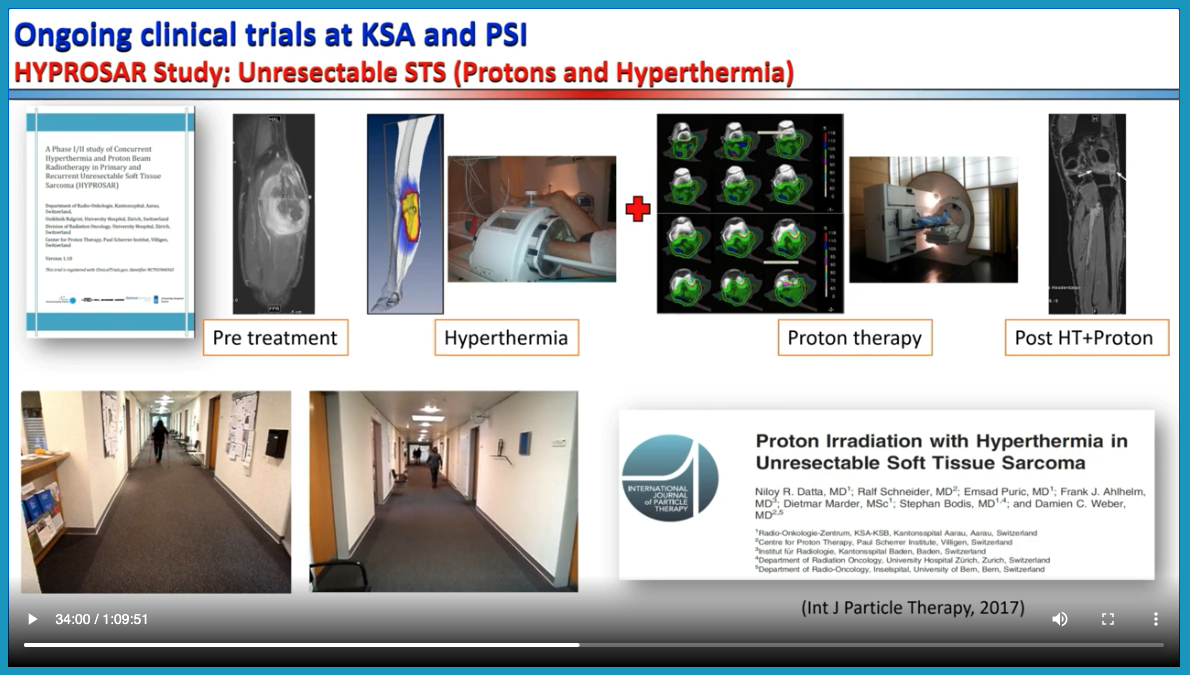
- Details
China is making significant strides in cancer treatment, positioning itself as a global leader in hyperthermia therapy. The Affiliated Zhongshan Hospital of Dalian University, a 2200-bed facility, is at the forefront of this movement. Specializing in advanced medical fields such as oncology radiotherapy, bone microsurgery, and hepatobiliary surgery, the hospital has recently launched a comprehensive Hyperthermia Center within its expansive 22-acre campus.

The Hyperthermia Center, established in March 2021, currently operates 13 hyperthermia devices and plans to expand to 20 by the end of 2024. This expansion will make it the largest oncological hyperthermia center in China, setting a new standard in cancer treatment.
 Pyrexar BSD-2000 Deep Regional Hyperthermia System
Pyrexar BSD-2000 Deep Regional Hyperthermia System
The center offers cutting-edge therapies that combine hyperthermia with chemotherapy and radiotherapy. The hospital is a center for research and Innovative treatments for advanced solid tumors. Results from several novel approaches aim to improve survival rates and enhance the quality of life for patients battling advanced tumors.

Acknowledgment to our distribution partners at Dalian Orientech Co., Ltd. for their hard work in making this center possible.














 Dr. Rodriques's program objective is to evaluate numerically the feasibility of focused heating in the minipig brain using the noninvasive MR-compatible brain applicator (Hal.o) and assess the feasibility of partial brain heating to design blood perfusion measurement experiments to improve the accuracy of temperature simulations using large mammal data. A full-scale prototype has been built and tested to verify heating performance.
Dr. Rodriques's program objective is to evaluate numerically the feasibility of focused heating in the minipig brain using the noninvasive MR-compatible brain applicator (Hal.o) and assess the feasibility of partial brain heating to design blood perfusion measurement experiments to improve the accuracy of temperature simulations using large mammal data. A full-scale prototype has been built and tested to verify heating performance.